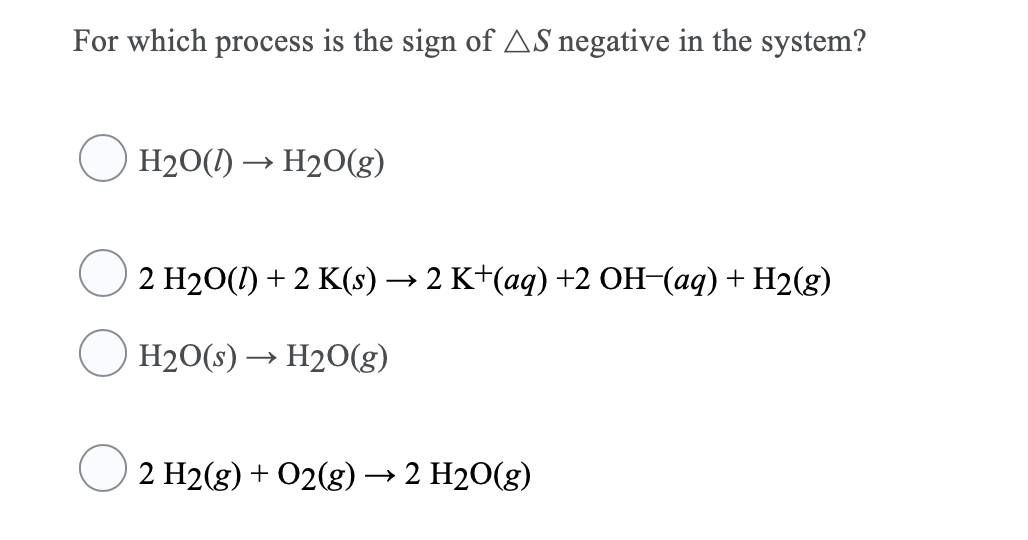SOLVED: Below is a proposed mechanism for the decomposition of H2O2. H2O2 + I– → H2O + IO– slow H2O2 + IO– → H2O + O2 + I– fast Which of the

The decomposition of H2O2 has a strong thermodynamic driving force 2H2O2→ 2H2O + O2(g) Δ H = - 99kj/mole,Δ s = + 69JK^-1 mole^-1 Addition of solution of KI causes H2O2 to

PSO Method for Fitting Analytic Potential Energy Functions. Application to I –(H2O) | Journal of Chemical Theory and Computation

In the system LaCl3(s) + H2O(g) + heat LaClO(s) + 2HCl(g) , equilibrium is established. More water vapour is added to restablish the equilbrium. The pressure of water vapour is doubled. The
![Comparison of the Raman spectra among [DEME][BF4]-H2O, [DEME][I]-H2O,... | Download Scientific Diagram Comparison of the Raman spectra among [DEME][BF4]-H2O, [DEME][I]-H2O,... | Download Scientific Diagram](https://www.researchgate.net/publication/258385483/figure/fig4/AS:320221761032204@1453358225281/Comparison-of-the-Raman-spectra-among-DEMEBF4-H2O-DEMEI-H2O-and-DEMEBr-H2O.png)
Comparison of the Raman spectra among [DEME][BF4]-H2O, [DEME][I]-H2O,... | Download Scientific Diagram