For the reaction : PCl5(g) PCl3(g) + Cl2(g) , if initial moles of PCl5 is 'x', a is the degree of dissociation and P is total pressure at equilibrium , then PPCl3 .

In the reaction PCl5 PCl3 + Cl2 the partial pressure of PCl3, Cl2 and PCl5 are 0.3 , 0.2 and 0.6 atm respectively at equilibrium. If partial pressure of PCl3 and Cl2

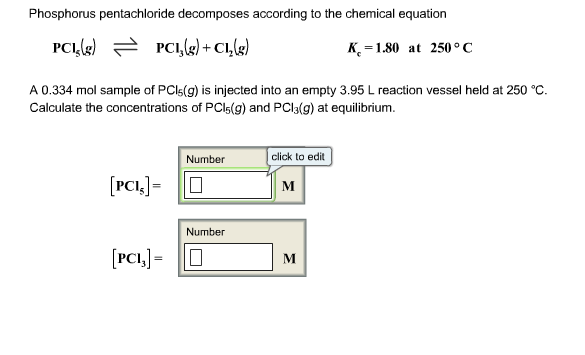
Phosphorus pentachloride decomposes according to the following chemical equation. PCl5(g) arrow PCl3(g) + Cl2(g); Kc = 1.80 at 250 degrees Celsius A 0.171-mole sample of PCl5(g) is injected into an empty 2.75

P+Cl2=PCl5 Balanced Eq.| phosphorus+ chlorine gas to form Phosphorus pentachloride Balanced Equation - YouTube
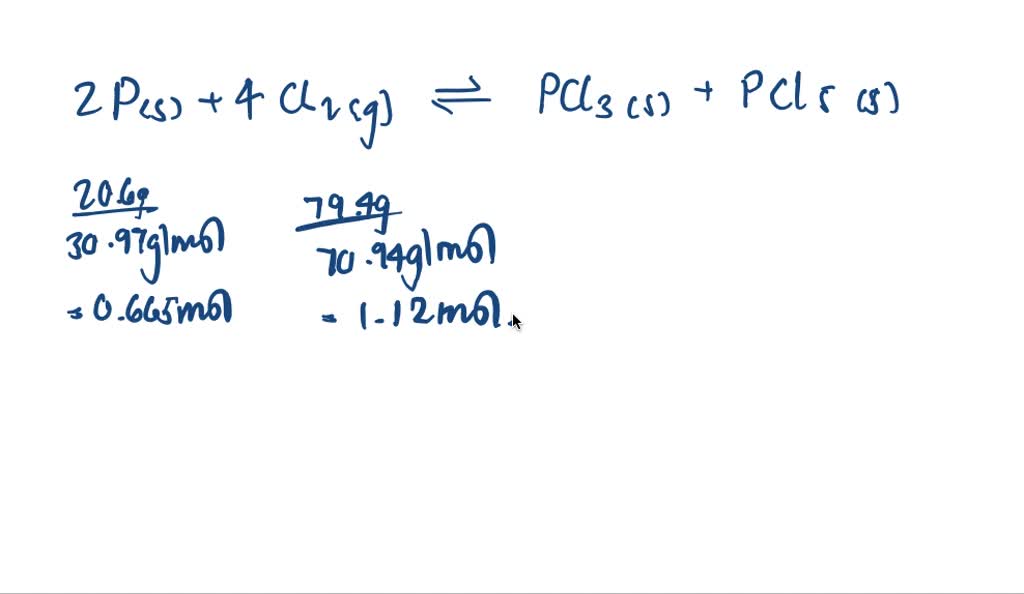
SOLVED:A mixture of 20.6 g of P and 79.4 g Cl2 reacts completely to form PCl3 and PCl5, which are the only products. Determine the mass of PCl3 that forms.
1. Pcl5=pcl3+cl2 Vapour density is found to be hundred when 1 mole of pcl5 is taken in 10dm3 flask at 300k. Thus, equilibrium pressure is : 1. 1.00 atm 2. 4.92 atm 3. 2.46 atm 4. 2.57 atm
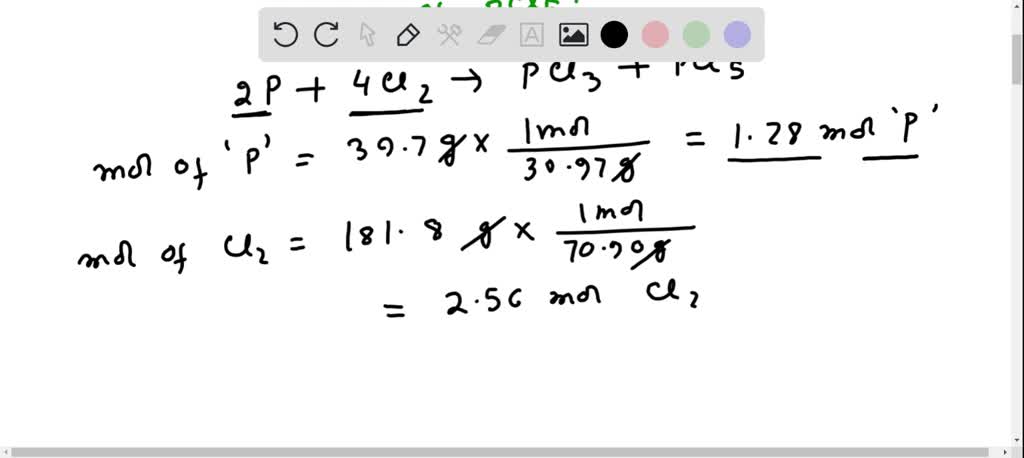
SOLVED: A mixture of 181.8 g of Cl2 and 39.7 g of P reacts completely to form PCl3 and PCl5. Find the mass of PCl5 produced. I really need help with this

In the reaction PCl5 PCl3 + Cl2 , the amount of PCl5, PCl3 and Cl2 at equilibrium are 2 moles each and the total pressure is 3 atm. The equilibrium constant Kp is :

P+Cl2=PCl5. Преобразуйте данную схему в уравнение реакции и вычислите массу фосфора необходимого - Школьные Знания.com
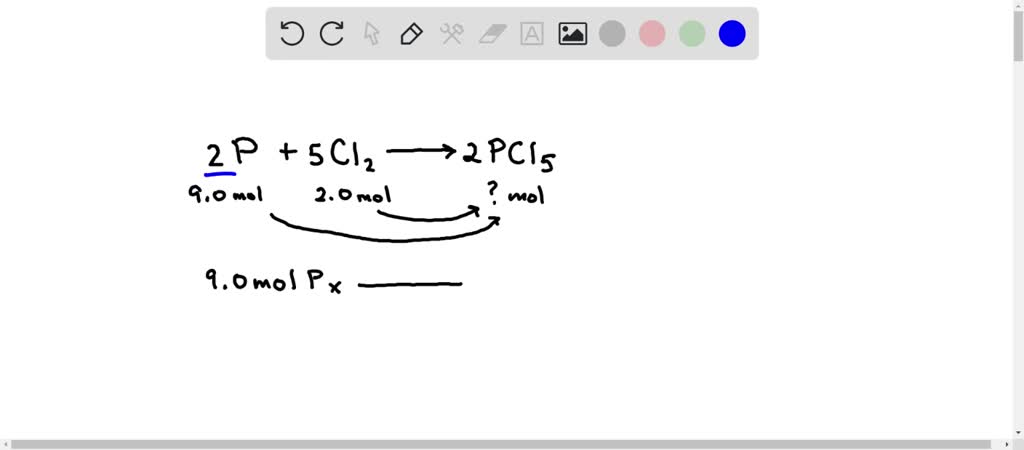
SOLVED: Solid phosphorus and chlorine gas react to form solid phosphorus pentachloride. Suppose you have 9.0 mol of P and 2.0 of Cl2 in a reactor. Calculate the largest amount of PCl5

SOLVED: Find the enthalpy for the reaction: PCl3( g)+Cl2( g)→PCl5( g) Given the reactions: P4( s) + 6Cl2( g) → 4PCl3( g) ΔH = -2439 kJ 4PCl5( g) → P4( s) +

How to balance P + Cl2 = PCl5|Chemical equation P + Cl2 = PCl5|reaction balance P + Cl2 = PCl5 - YouTube